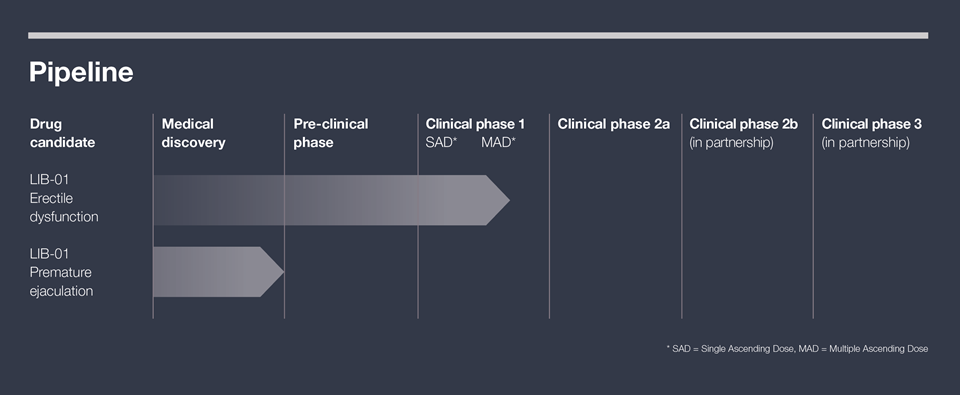
The drug candidate LIB-01
The overall goal is to develop LIB-01 into a registered drug on the world market, that treats erectile dysfunction and premature ejaculation better than currently available drugs.
The first clinical trial of LIB-01 in humans started in August 2023. The nonclinical development program required prior to start of clinical trials was completed during the spring 2023. The pharmacology studies showed that LIB-01 has a significant pro-erectile effect with a long duration and the toxicology studies showed a good safety profile. Within the LIB-01 development program, process development and manufacturing are also carried out to ensure availability of the drug substance. The drug substance together with a formulation, constitute the drug product that is used in nonclinical studies and clinical trials.