Patent
Dicot has a global IP strategy with a long term perspective.
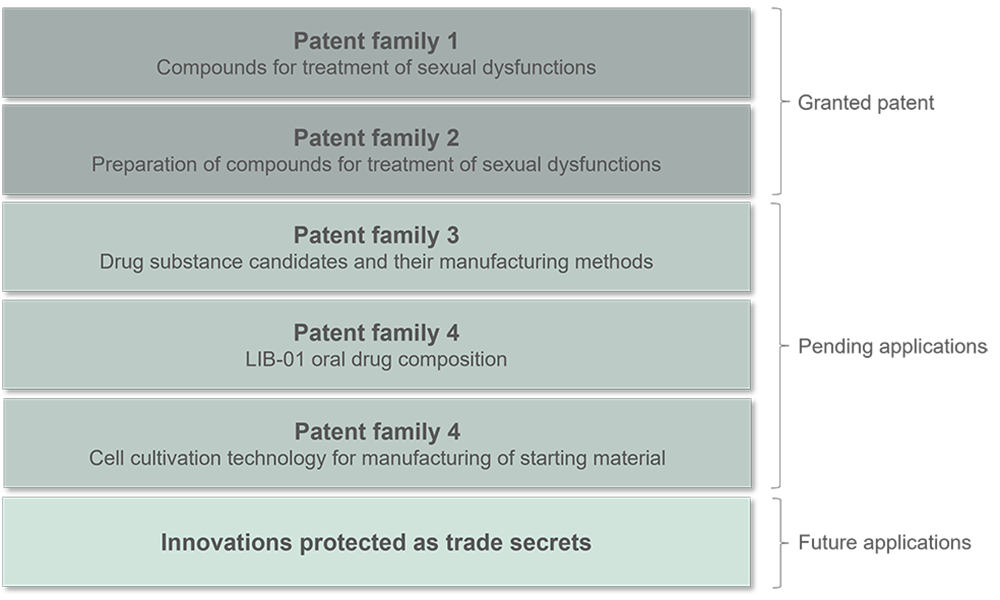
Dicot has existing patent protection by two already granted patent families. In addition, the company has applied for two new patent families and intend to apply for further patent protection based on its active ongoing IP work. Today Dicot has market exclusivity on key markets until 2033. The most recent patent applications intend to provide patent protection until 2042 and 2043 respectively. Dicot holds additional patent opportunities documented and protected as trade secrets, in order to convert these into patents at the right point of time. The patent strategy is global and important to maximize the assets and value of the company. Dicot has a pronounced partnering strategy to out-license LIB-01 when clinical data is at hand.
Dicot´s IP assets